NEW We've just released new features
Unified eQMS solutions.
Built For Compliance, Customized For You.
Designed exclusively for Medical Devices by Regulatory & QMS experts






Gaps in Current practices and existing eQMS systems.

1. Errors & Oversight
Prone to human-errors and oversight:
Manual entries, scattered documents, and repetitive tasks create multiple points of failure, leading to data discrepancies and non-compliance.

2. Disconnection
Disconnected individual modules with no real-time cross-departmental sync
Existing eQMS solutions lack the traceability and inter-departmental communication that a software solution should provide.

3. Audit & Inspection Issues
Poor Audit/Inspection Readiness
eQMS softwares working on disconnected modules often lead to departmental silos and lack of traceability leading to non-conformances.

4. Time & Resource Limits
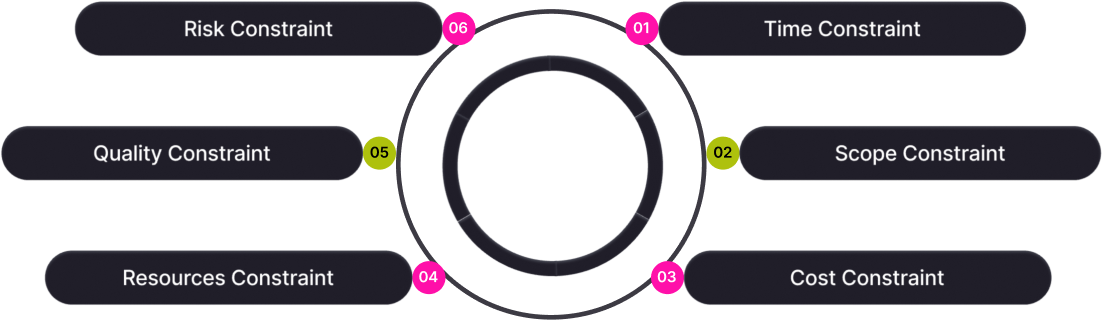
Resource and time constraints
Startups and small-to-mid-sized manufacturers often lack dedicated QA/RA staff. Startups spend 6-8 months creating a QMS and still risk audit failures.
How Regtech can empower you
Unlike current eQMS tools that operate with disconnected modules, siloed data, and no real-time cross-departmental sync often requiring manual cross-linking and leaving teams unprepared for inspections, Regtech delivers a unified, audit-ready platform built for seamless collaboration and compliance that mirrors the cross-functional reality of ISO 13485. Rather than isolating workflows into rigid modules, Regtech offers seamless, logic-driven processes that unify all departments within a single intelligent system.
All Departments. One Seamless Platform.
Experience true inter-departmental connectivity through a unified QMS dashboard. Regtech brings your daily operations, data, and decisions into one integrated space — empowering faster, smarter, and fully compliant execution. Customize your dashboard by department, role, or focus area, so you always see what matters most.
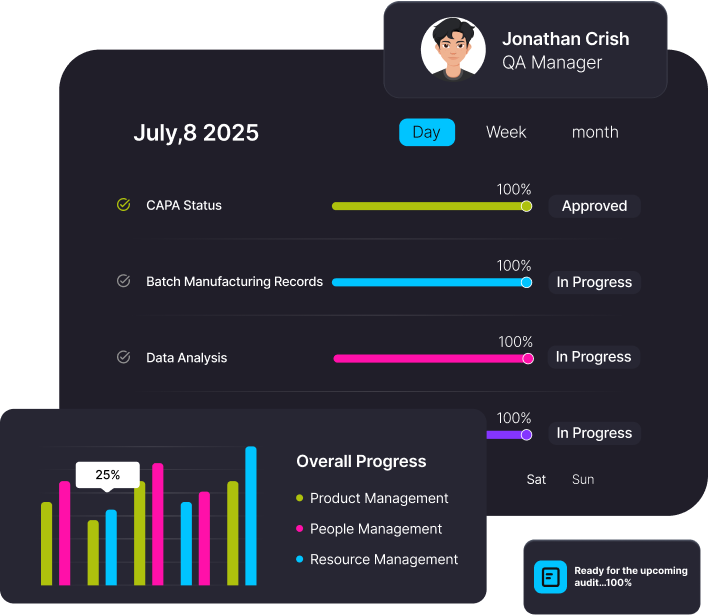
Automated Workflows with No Process Delays & Full Audit Traceability
Regtech replicates your SOPs with precision ensuring no step is skipped, no action goes unrecorded.
Every process flows through a defined, compliant path, automatically capturing non-conformances, triggering CAPAs, and driving continual improvement.
Stay audit-ready with clean, searchable audit trails.
Monitor & Maintain Your QMS — Anytime, Anywhere
With Regtech, compliance doesn’t stop when you step away. Access your Quality Management System in real-time from any location. It empowers leadership to make strategic decisions based on live data, without being on the shop floor.
Manage tasks smartly
Get actionable alerts & notifications
Whether a document is awaiting your approval or if a production batch is ready for Quality Control testing or you have CAPAs that are open, you get automated, role-specific notifications to ensure you act on tasks that truly need your attention.
Product features that will enhance team productivity
Smarter Tools for Every Team Member

Production
Smart production planning, auto-generated material requisitions, and automated inter-departmental register updates. Complete transparency, from input to output. Non-conforming devices are automatically flagged in the NC Register so that you can easily categorize items as “Rework or Reject” based on your assessment. Spilt your BMRs with Regtech’s automated calculations and batch adjustments

Quality Control
Automated AQL sampling, schedule-based calibration prompts, and automated deviation reporting against acceptance criteria. Complete visibility with real-time non-conformance logs so you can quickly track issues as they arise. Plan your QC activities based on real-time tracking of production processes and ensure compliance at every stage. Automise reporting and stay ahead with faster corrective actions.

Quality Assurance
Get auto-reminders for validations and alerts for line-clearance. Quickly generate quality procedures using basic information, with document numbers and revisions automatically applied according to set controls. Track all open tasks from audits, reviews, and quality processes, and turn them into an organized, actionable To-Do list for easier management and faster follow-up.

Human Resources
Automatic MCQ evaluation and result generation, ePapers specific to each training session, and retraining triggers for failed trainees ensure learning gaps are quickly addressed. Attendance sheet accessibility windows keep records up to date. A built-in organogram makes it easy to assign access, manage roles, and track ownership across teams seamlessly and efficiently.

Top Management
Live dashboards, manufacturing trends, data analysis, KPIs, audit performance, post-market feedback, and customer complaints—all at a click without needing to be on the shop-floor. Get instant insights, track performance metrics, and identify issues in real time so you can make informed decisions and improve operations from anywhere, anytime.

Purchase
Procurement process at your fingertips. From analysing real-time supplier performance data to tracking purchase order lifecycles, you can ensure cost-effective, on-time, and quality-controlled sourcing. Manage your supply chain with complete transparency, monitor vendor compliance, and make data-driven decisions.
“Get role specific notifications & alerts using Regtech”
CAPA closed
Approval awaited
Calibration Due
Initiate Material requisition
IPQC completed
Review Audit plan
Finished Goods Approved
SOP updated
Need analysis
Review Audit plan
Our process
Our Simple, Smart, and Scalable Process
We design, develop, and implement automation tools that help you work smarter, not harder
Smart analysing
We analyse the roles undertaken by the organisation, systems implemented, processes defined, manufacturing flow and lay down a customised plan to provide specific solution. Easily adapt the system to your organization’s size and needs, whether a startup or multinational enterprise
Builds your smart eQMS
Our Team then builds your smart eQMS tailored to your systems and processes.
Seamless integration
Our solutions are compatible and seamlessly integrate with ERP, LIMS, HRMS, and other enterprise systems via open APIs. Enable secure collaboration across locations with role-based permissions and cloud access.
Software implementation
Once the software is ready, our QA experts and IT developers ensure a smooth transition and implementation on-site for its effective and optimised usage.
Continual improvement via regular maintenance and upgrades
Our evolving software is continuously upgrading itself based on user experiences, updated regulations and standards and state of the art practices offering our client an opportunity to evolve with it.
Our MOAT
Why choose us over others?

- First ever Unified eQMS
- Customisable to the simplest daily tasks
- Fast setup with built-in templates
- Real-time reporting and analysis
- Automated task reminders
- Inter-linked Inter-departmental processes
- Systems are always audit-ready
- Fragmented modules
- Limited customisation
- Slower execution
- Limited or delayed reporting
- Only for data entry
- Lack of departmental connectivity
- Poor traceability leading to audit failures
Trusted by: Brands & testimonials
Daniel Kim
Operations Lead at Flowbyte
“Truly impressive. The AI assistant is fast, accurate, and blends into our daily ops without friction.”
Priya Mehra
CTO at Brightstack Labs
“Game-changer. Automation flows run flawlessly. Our team now focuses only on what really matters.”
Elena Rodriguez
Product Manager at Nexora
“Smooth setup. Their system replaced three tools. We saw improvements in just the first week.”
Marcus Thompson
Marketing Director at OrbitShift Director
“Surprisingly simple. The AI adapts quickly. Our campaigns are now running 2x more efficiently.”
Sarah Wong
Analytics Manager at Corelink
“Huge time-saver. Data is better organized. The insights we get now are actionable and fast.”
Ravi Shah
COO at PixelNest Solutions
“Very intuitive. No fluff, just performance. Our internal processes finally feel under control.”
Ready to Automate Smarter?
Let’s Build Together
Schedule a Call and Begin Automating
demo@mavenregtech.com
Frequently Asked Questions
Find quick answers to the most common support questions
Still Have Questions?
Still have questions? Feel free to get in touch with us today!
What is an eQMS and why do medical device companies need one?
An eQMS (Electronic Quality Management System) helps medical device companies manage compliance with industry regulations like ISO 13485 and FDA 21 CFR Part 11. It centralizes quality processes like CAPA, document control, audits, training, and risk management—making your systems audit-ready, traceable, and efficient. Without an eQMS, you’re stuck juggling spreadsheets, emails, and siloed tools that slow you down and put compliance at risk.
How is RegTech different from generic QMS software?
Can startups use RegTech, or is it only for enterprise companies?
Does RegTech support multisite and multiuser access?
What types of processes can you automate?
We specialize in automating repetitive workflows across operations, marketing, sales, and customer support using AI and custom logic.
Do I need technical knowledge to use your service?
We specialize in automating repetitive workflows across operations, marketing, sales, and customer support using AI and custom logic.
Can you integrate with our existing tools?
We specialize in automating repetitive workflows across operations, marketing, sales, and customer support using AI and custom logic.
How long does implementation take?
We specialize in automating repetitive workflows across operations, marketing, sales, and customer support using AI and custom logic.
Is your AI secure and compliant?
We specialize in automating repetitive workflows across operations, marketing, sales, and customer support using AI and custom logic.