Stay ahead with insights that matter
Our blogs section is where regulatory expertise meets technology. From evolving ISO 13485 requirements to practical tips on digital QMS adoption, explore thought leadership, industry updates, and actionable strategies for medical device compliance.
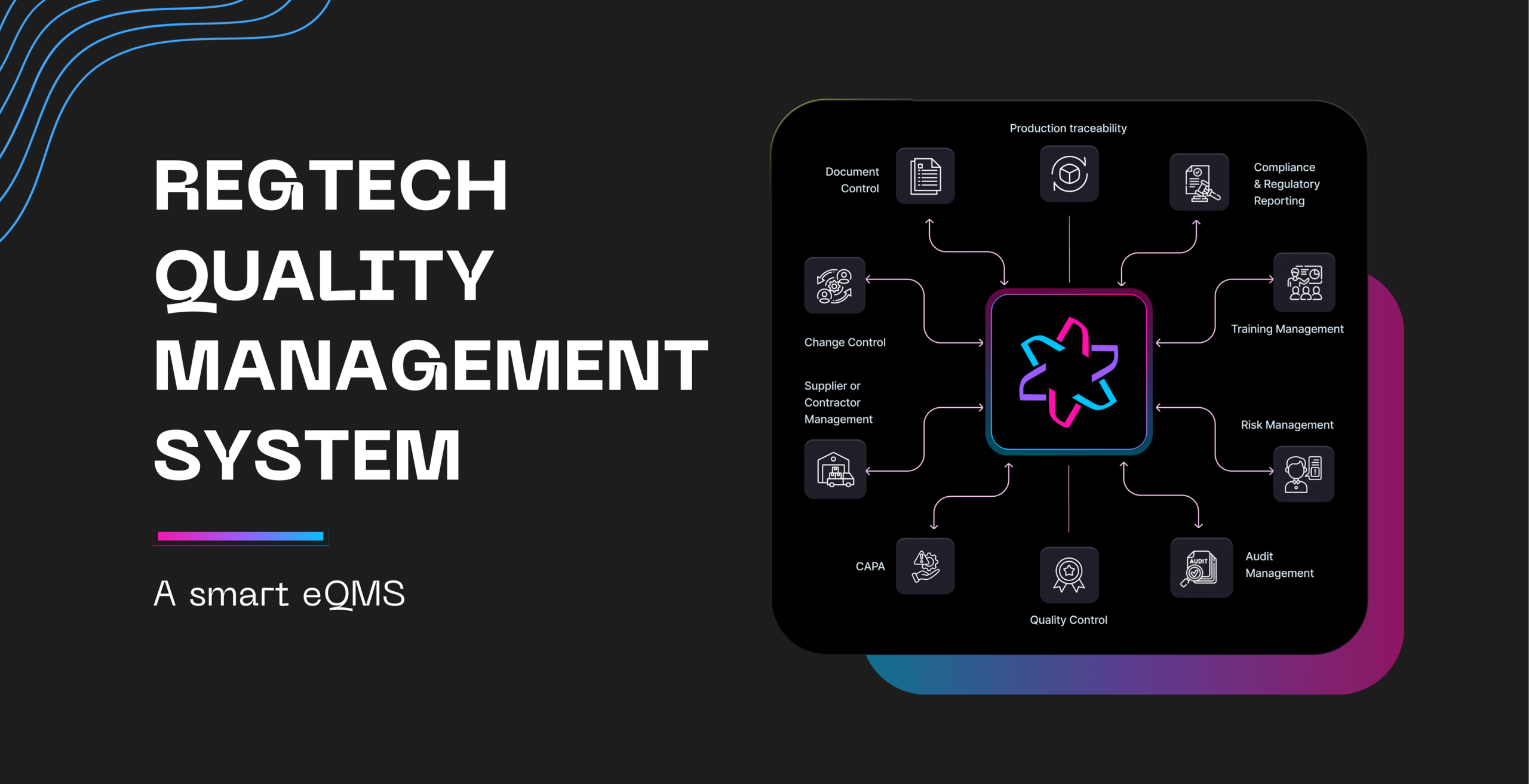
Introduction: The Cost of Compliance Uncertainty For medical device manufacturers, the Quality Management System (QMS) is the single most critical
The Problem: Your QMS is Logging Data, But Missing Dialogue In regulated medical device manufacturing, quality work is…
In the highly regulated world of medical devices, managing risk (per ISO 14971) is essential, but it remains…
In the fast-paced world of medical device manufacturing, quality isn’t just a goal—it’s a non-negotiable requirement. While many…
Introduction: The Cost of Compliance Uncertainty For medical device manufacturers, the Quality Management System (QMS) is the single most critical asset. A robust, well-maintained QMS …
The Problem: Your QMS is Logging Data, But Missing Dialogue In regulated medical device manufacturing, quality work is inherently cross-functional. A single CAPA (Corrective and …
In the highly regulated world of medical devices, managing risk (per ISO 14971) is essential, but it remains a bottleneck due to outdated tools. For …
Ready to Automate Smarter?
Let’s Build Together
Schedule a Call and Begin Automating
demo@mavenregtech.com