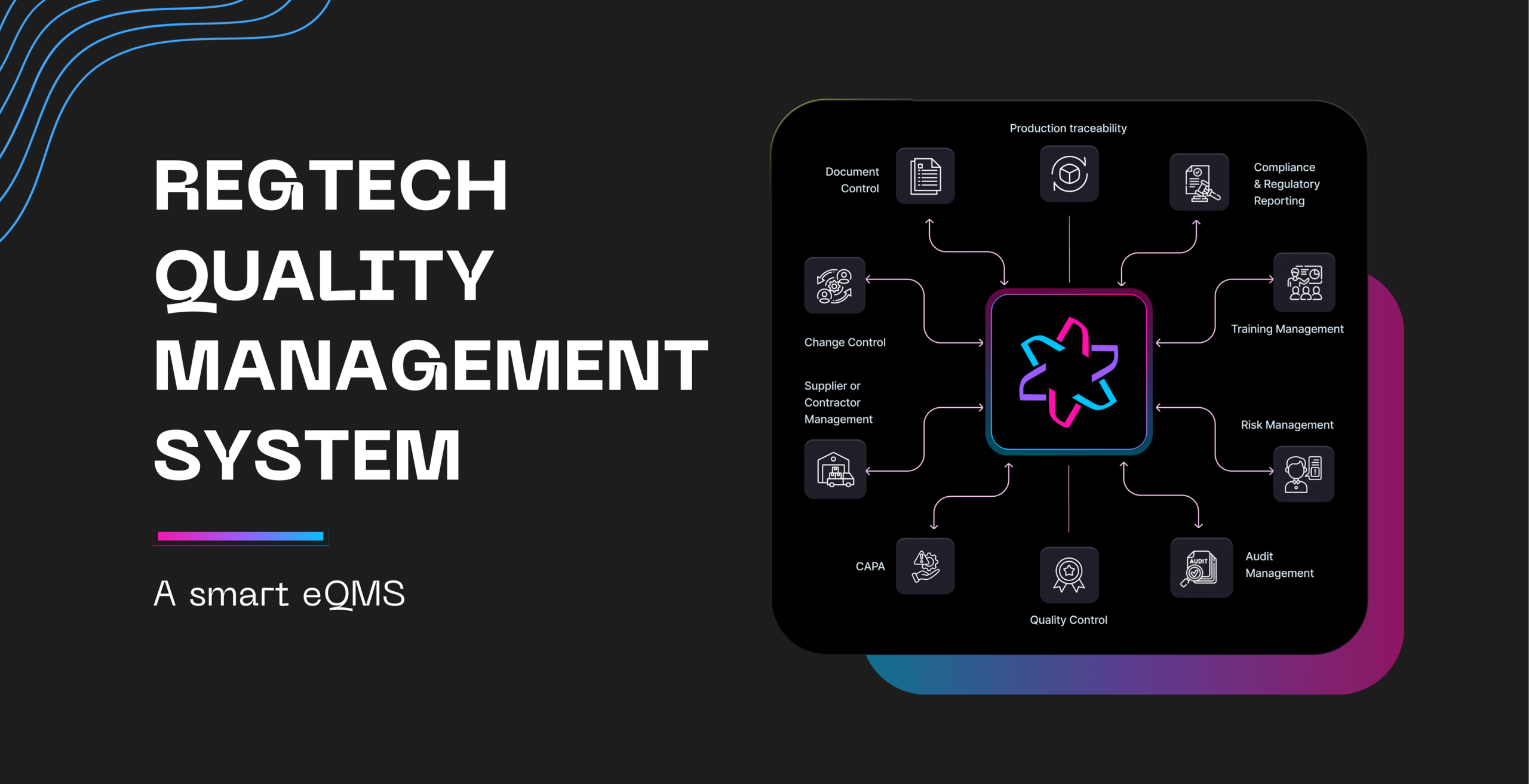
Introduction: The Cost of Compliance Uncertainty
For medical device manufacturers, the Quality Management System (QMS) is the single most critical asset. A robust, well-maintained QMS is the input that acts as a foundation to any global certification and registration. Yet, many organizations are still burdened by legacy, paper-based, or fragmented systems that cause the same three chronic issues for the Quality Assurance (QA), Regulatory Affairs (RA), and Top Management:
- Costly Delays: Slow processes and audit findings derail time-to-market.
- Audit Anxiety: Lack of real-time traceability creates panic during ISO 13485 and FDA audits.
- Inefficient Teams: High-value QA/RA staff waste time chasing signatures and managing spreadsheets.
Regtech is the modern, cloud-based eQMS built by medical device experts specifically to eliminate these bottlenecks. We don’t just digitize your paperwork; we automate your path to compliance, guaranteeing a more efficient, profitable, and audit-ready operation.
Product Highlights
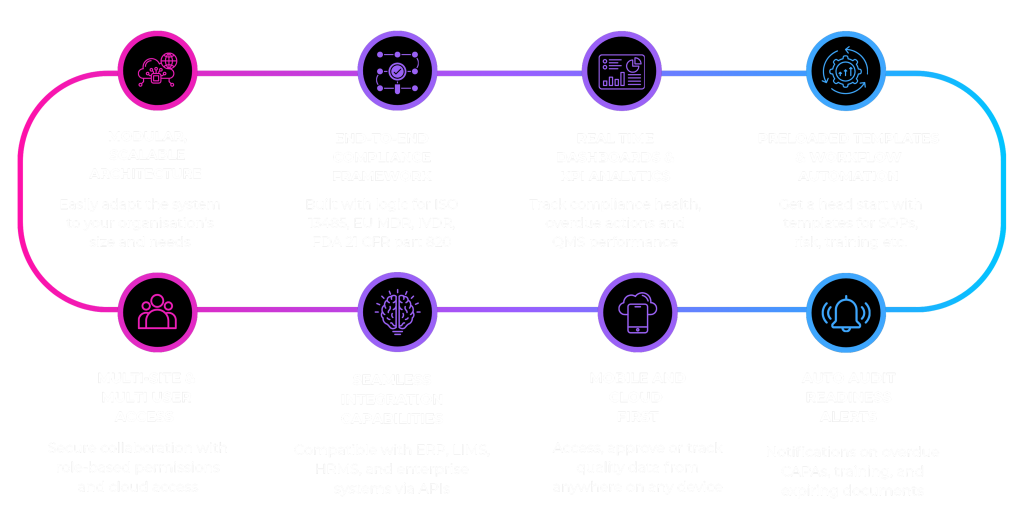
The Proof is in the Numbers
The value of RegTech moves beyond “easier compliance” and directly impacts your financial health and time-to-market. Leading manufacturers in the medical device and pharmaceutical industries have reported the following verifiable benefits after adopting a robust eQMS:
- Accelerated Time-to-Market: By automating document review, approvals, and design control processes, companies report a 25% to 40% reduction in the product development lifecycle.
The result: Your innovations reach patients, and market revenue comes in faster.
- Reduced Cost of Non-Quality: By transitioning from a reactive to a proactive quality system, manufacturers experience a significant decrease in defects, rework, and scrap. Industry data consistently shows that a mature QMS can lead to a 15% to 30% decrease in the Cost of Quality.
The result: Higher manufacturing yields and better margins.
- Streamlined Audit and Inspection Response: Eliminating the “audit scramble” saves critical staff time. Companies have quantified this, reporting over 50% less time and effort required to prepare for and host external regulatory inspections.
The result: Highly paid QA/RA staff can focus on value-add activities instead of administrative chasing.
- Enhanced Organizational Scalability: Manual systems choke growth. RegTech solutions are built to scale seamlessly from a single manufacturing site to global, multi-site operations, all while enforcing a single, standardized Quality System.
The result: The quality system is an accelerator for business expansion, not a brake.
| Pain Point | Solution |
|---|---|
| Market Risk | Guaranteed Predictability: Regulatory unpredictability is a drain on resources. Regtech ensures your processes are standardized and compliant with ISO 13485, FDA CFR Part 820, and MDSAP from Day 1, de-risking every device launch. |
| Lost Revenue | Accelerated Time-to-Market: By automating document control, training, and change management, we eliminate the common administrative roadblocks that cost you revenue. Spend less time in compliance review and more time selling. |
| System Cost | Single Source of Truth: Replace disparate systems, spreadsheets, and file shares with one integrated eQMS. This drastically reduces IT overhead, data integrity risks, and training complexity. |
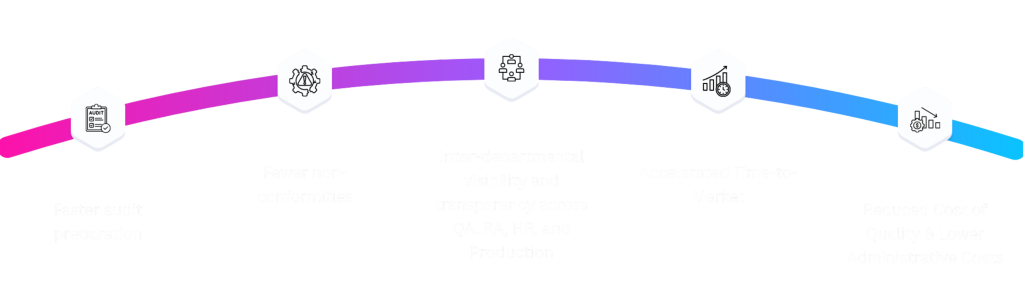
How to get started with our eQMS: A client onboarding roadmap
As your dedicated eQMS provider, our goal is to ensure a seamless, compliant, and value-driven transition from your current system to our fully validated electronic platform. We follow a five-phase roadmap designed for rapid deployment and maximum user adoption.
Phase I: Discovery and Scoping
- Initial Assessment: We start with an in-depth session to understand your current Quality Management System (QMS) structure, documented procedures, and regulatory requirements (e.g., FDA 21 CFR Part 820, ISO 13485, MDSAP, EU MDR).]
- Define key milestones based on roles undertaken by the organisation: Based on the activities performed by the organisation, the customisation of the eQMS platform can be defined, and key milestones can be identified along with the identification of key departmental stakeholders who will work alongside our implementation specialists.
Phase II: Configuration and Validation Planning
- Workflow Mapping: Our team maps the digital workflows within the eQMS to mirror your existing, approved Standard Operating Procedures (SOPs). This includes customizing review cycles, approval routes, and notification triggers.
- System Configuration: We configure user roles, permissions, data fields, and reporting dashboards specific to your organization’s structure.
- Validation Strategy (IQ, OQ, PQ): We deliver our pre-executed Installation Qualification (IQ) and Operational Qualification (OQ) documentation. We then work with your QA team to tailor and execute the Performance Qualification (PQ) protocols, ensuring the system functions reliably in your environment and fully meets regulatory requirements for electronic records (21 CFR Part 11 Compliance).
- Data Migration Plan: We develop a targeted plan for migrating essential, current documents and active records, minimizing disruption while maintaining data integrity.
Phase III: Training and Pilot Deployment
We focus on user readiness and real-world testing to ensure a smooth Go-Live.
- Role-Based Training: We provide comprehensive training tailored to specific user functions (e.g., Administrator, Document Controller, End-User). Our goal is high user proficiency before launch.
- Pilot Program: We launch the configured eQMS with a small, manageable team to test the end-to-end process in a live environment. This helps us catch and fine-tune any minor workflow adjustments.
- Final Acceptance: Following successful completion of the Pilot and PQ, we obtain your formal sign-off on the configured and validated system.
Phase IV: Go-Live
The system is deployed across your organization, and the transition is complete.
- Official Launch: We set the official Go-Live date, transitioning all core quality processes from your legacy system to the new eQMS.
- Hypercare Support: For the first 30 days post-launch, our support team provides accelerated, dedicated assistance to quickly resolve any user questions or minor technical issues, ensuring high adoption rates.
- Phase V: Ongoing Partnership and Compliance Contract
Our relationship continues beyond deployment to ensure your system remains compliant and continually adds value. Our maintenance contracts address the following:
- Maintenance of Validation Status: We handle all required validation updates for our regular software releases and upgrades, significantly reducing your compliance burden.
- New Module Rollout: We support the phased rollout of additional modules (e.g., Supplier Management, Risk Management) as your business needs evolve.
- Regulatory Updates: As a RegTech partner, we ensure our platform evolves with regulatory changes, keeping your QMS automatically aligned with the latest requirements.
Conclusion: Transform Compliance into Competitive Advantage
The future of manufacturing is digital, integrated, and intelligent. RegTech is not just an IT project; it is a business optimization strategy that secures your compliance while unleashing your efficiency.
The choice is simple: Continue to bear the operational drag and financial risk of fragmented, paper-based compliance, or embrace the future and transform your regulatory burden into a powerful, automated engine for quality, speed, and sustained growth.
Your Next Audit Can Be Stress-Free
Stop letting your QMS slow down your market entry. See why leading medical device manufacturers choose Regtech to achieve seamless ISO 13485 compliance and accelerate their launch timelines.
- Eliminate audit stress with 1-click traceability
- Reduce CAPA cycles by 40%
- Maintain 100% workforce compliance
- Transform supply chain risk into a partnership